Debate on Abiogenesis: Dr. James Tour versus Dave Farina: Part 1 – The Formation of Peptides
Abiogenesis, the idea that life sprang from unguided chemistry, continues to be a central topic in the creation/evolution debate. On May 19, 2023, Dr. James Tour, a Messianic Jew, organic chemistry professor at Rice University, and well-known associate of intelligent design advocates, debated Mr. Dave Farina, aka “Professor Dave,” on the question of the origin of life (Fig. 1). 1 The debate was entitled “Are we clueless about the origin of life?” The debate was held in Keck Hall on the Rice University campus before about 2800 people viewing in person and online. 2 The debate, including a Q&A session, lasted about two and a half hours. As of July 8, 2023, the debate had been viewed 135,019 times on YouTube accompanied by 20,798 comments. The YouTube recording of the debate has been professionally annotated so that the dialog and references cited can be clearly followed.
NOTE: Click on images to expand them.
The purpose of this article is to convey and comment upon some of the scientific arguments made during the debate. Farina acted as though many of the problems facing abiogenesis have been solved and cited numerous papers to support these claims. Tour’s position was that there are still many problems and that article titles are often misleading. Tour, in his opening segment, listed five problems for abiogenesis (see Fig. 1) which, he claimed, the scientific community has yet to sufficiently address:
- the formation of polypeptides,
- the formation of polynucleotides,
- the formation of polysaccharides,
- the origin of the specified information in biology, and
- assembly of a living cell.
Tour’s list will be used as the framework for a series of articles over the next few months. Secular timescales (deep time) will be assumed for the sake of argument. The formation of peptides will be covered in this first installment.
The debate did not allow for a detailed discussion of most of the points made. In this essay, I have attempted to use some of the journal articles cited during the debate to determine if the particular claims that abiogenesis is supported are valid. Dr. Tour, to his credit, said during his opening statement that he would be considering the data, and not the hype, of the papers Farina put forth.
For a particular scenario of abiogenesis to be credible, it must be consistent with the supposed geochemistry of the early Earth and the known laws of chemistry. Origin of life (OOL) laboratory experiments must not introduce unnatural events such as the purification of reaction products or the use of chemicals that could not have been available, nor can they employ unrealistic reaction conditions such as high reactant purities, organic solvents, complex order of combining chemicals, improbable temperatures, unlikely pH, extremely high reactant concentrations, unlikely atmospheres, improbable oxidation states of metals, etc. The more control over the conditions of an experiment that is required for its success, the less likely the experiment is representative of realistic prebiotic conditions. The leading theory of abiogenesis is the “RNA world” hypothesis. This hypothesis holds that a self-replicating RNA molecule was the first “life” that then eventually evolved into modern biochemistry. There are RNA molecules called ribozymes that are known to be able to carry information as well as perform as catalysts. The question of the RNA world arose in the debate. For the RNA world to be credible, one must show that a self-replicating RNA molecule could have arisen from unguided chemistry. As we shall see, the available data do not support this. 3 To the extent that successful reaction outcomes rely on experimenter interference, abiogenesis is discredited. For much of the abiogenesis work cited here, the task will be to “follow the information,” that is, to identify where the information in a chemical system or molecule came from – unguided chemistry, functional biomolecules in extant life, or the creativity of chemists!?
Some of the discussion here will be semi-technical, but an attempt has been made to use language that will hopefully communicate the main ideas to a general audience.
For the sake of time, Dr. Tour conceded the issue of homochirality. Homochirality refers to the specific threedimensional structures of the molecules that make up living things and is a critical aspect of biochemistry. Homochirality is one of the major unsolved difficulties for abiogenesis. Despite Tour’s concession, homochirality will be discussed here as appropriate.
Formation of Polypeptides (proteins and enzymes)
As a brief background, proteins are polymers made from twenty unique amino acids (AAs) joined together with amide bonds. Almost all AAs in proteins have a “lefthanded” or “L” three-dimensional structure. The amide bonds in proteins can be hydrolyzed by water. Also, the three-dimensional L structures of AAs can be scrambled or racemized in water. Racemization in extant proteins is often associated with disease. 4 On average, it is estimated that functional proteins can only tolerate 5% racemization before function is diminished or lost. 5 Studies have determined that the half-lives towards racemization of free AAs in neutral (pH 7) water at 25° C (77° F) can vary from a century to thousands of years, but the half-lives are much shorter for AAs incorporated into proteins. Racemization rates also increase in the presence of metal ions, higher temperatures, or at different pH. 6 Indeed, given the likely very low concentrations of AAs in the early oceans, the rate of racemization would probably have exceeded the rate of polypeptide formation so that no homochiral polypeptides could have ever formed. 7
Dr. Tour’s first point (Fig. 2) was that the incorporation of the amino acid lysine into a protein using only the \(\alpha\)-amino group would be a problem under prebiotic conditions. The structure of the amino acid lysine is shown in Figure 3. Lysine contains two amino groups, each of which could form amide bonds with other amino acids.
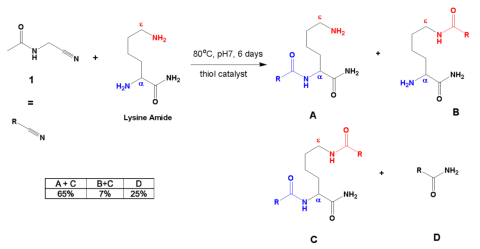
Extant proteins contain \(\alpha\) linkages exclusively (Fig. 3). Proteins in living organisms are assembled in ribosomes from the twenty different amino acids using RNA and protein enzymes. The ribosome can make polypeptides which only have \(\alpha\) linkages. Undirected chemistry produces both \(\alpha\) and \(\epsilon\) linkages (Fig. 3). Farina asserted the problem of selectivity for only \(\alpha\) linkages under prebiotic conditions had been solved. Tour disagreed. Farina cited a 2022 paper by Singh (Fig. 4) where the investigators combined acetylated \(\alpha\) aminonitriles (1) with the amide of lysine in the presence of a thiol catalyst to form a mixture of products, some derived from lysine (A–C), and the hydration product of the \(\alpha\) aminonitrile (D). 8
The table inserted in Figure 4 displays the best results obtained in terms of the largest observed ratio of \(\alpha\)/\(\epsilon\) products formed. The data are reported as in the paper.
The percentages are yields determined by nuclear magnetic resonance using an internal standard. A+C represents all the products with an \(\alpha\) linkage while B+C represents all the products with an \(\epsilon\) linkage. C is included in both sums since it contains both the \(\alpha\) and \(\epsilon\) linkages. In Table 3 of the paper, the authors reported that the overall \(\alpha\)/\(\epsilon\) ratio for this reaction was 9.3. This ratio may seem high, but most extant proteins contain only a linked lysine. 9 Hydration product D was formed in 25% yield. The reaction used 0.2 M concentrations of pure acetyl aminonitrile 1 and the lysine amide with 0.3 equivalents of the thiol catalyst (Fig. 4). These are concentrations that are too high for an early Earth. The reaction does not work without the presence of a thiol catalyst with low acidity. The \(\alpha\) selectivity was diminished at pH 9. Ordinary amino acids do not easily make polypeptides and the \alpha\ aminonitriles only work when acetylated, so only acetylated \alpha\ aminonitriles will successfully make polypeptides at neutral pH. There was evidence of racemization under the same or similar reaction conditions as shown in Figure 4 when the starting amino acid amide contained more than one chiral center. 10 This means the specific three-dimensional structures of the amino acids were lost during the reaction. This result alone makes this route to peptides implausible as a prebiotic pathway since extant proteins contain amino acids with specific threedimensional structures (all are Lisomers). The preparation of acetyl aminonitrile 1 in Figure 4 was often accomplished using storebought aminonitriles that were acetylated with thioacetate at pH 9 in the presence of K3[Fe(CN)6]. 11 However, recent studies have shown that a significant accumulation of thioacetate under a broad range of possible conditions on the prebiotic Earth is geochemically implausible. 12 Hence, the best result–which was still lacking—depended upon specific starting materials (acetylated \(\alpha\) amino acids), high reactant concentrations and purities, a specific thiol catalyst, a specific pH range, starting materials that were storebought or made with conditions unavailable on the early Earth, and a procedure that resulted in the racemization of
the amino acids in the products. So, this procedure was not only unlikely to represent prebiotic conditions but introduced another problem (racemization) which is of even greater concern.
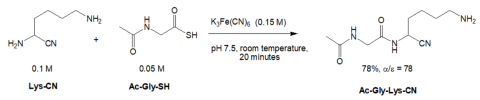
A second paper relevant to this discussion (not mentioned during the debate) was published by Thoma and Powner in 2023. 13 Under their best conditions, they observed an \(\alpha\)/\(\epsilon\) linkage ratio of 78:1 (Fig. 5).
The \(\alpha\) selectivity was sensitive to pH. 14 The same reaction conducted at pH 8.5 afforded an \(\alpha\)/\(\epsilon\) linkage ratio of only 7.5:1, a significant decrease. Above pH 8.5, the an \(\alpha\)/\(\epsilon\) linkage ratio was ≤ 2.5:1. The authors suggested the high \(\alpha\) selectivity seen at pH 7.5 (Fig. 5) was due to the differences in the basicities of the \(\alpha\) and \(\epsilon\) groups in Lys-CN; at pH 7.5, the \(\epsilon\) amino group would have been protonated but the \(\alpha\) amino group would not have been protonated favoring it to form an amide bond. They found that if unmodified lysine or lys-NH2 was used instead of Lyn-CN, the high \(\alpha\) selectivity was lost.
To prepare Lys-CN they employed a multistep procedure using various organic solvents, temperature extremes, controlled order of addition of reagents, non-prebiotic chemicals, and so forth. 15 Clearly, these procedures do not represent the chemistry available on the supposed early Earth. Several papers in the literature say that the prebiotic origin of amino acids may have been a reaction between aldehydes with ammonia and then cyanide (Strecker reaction), 16 but the papers discussed above did not use this chemistry to prepare \(\alpha\) aminonitriles.
Ac-Gly-SH (Fig. 5) was prepared from Ac-Gly-SNH2 in dimethyl sulfoxide and water at pH 9 at 60° C. 17 Ac-Gly-SNH2 was made from Ac-Gly-CN at pH 9. Ac-Gly-CN was made by treating GlyCN with AcSH, a compound unlikely to have been present on the early Earth. Hence, the thioacid Ac-Gly-SH (Fig. 5) would not likely be present on the early Earth for the same reasons thioacetate (mentioned above) would not have been present. 12
Look for part 2 in the series this fall.
- 1The debate is available on YouTube: Are we clueless about the origin of life? https://www.youtube.com/watch?v=pxEWXGSIpAI&t=4s Accessed 2023 Jul 17
- 2Shad N (2023 Jul 17) Rice’s James Tour and YouTuber ‘Professor Dave’ debate the origins of life. The Rice Thresher. https://www.ricethresher.org/article/2023/05/rices-james-tour-and-youtuber-professordave-debate-the-origins-of-life Accessed 2023 Jul 17
- 3For a recent book discussing many of the problems for the RNA world hypothesis, see Tan L, Stadler R (2020) The Stairway to Life. For a comprehensive review of the book, see Reynolds D (2020 Jul) Review of The Stairway to Life: An Origin of Life Reality Check. https://www.tasccreationscience.org/sites/default/files/2021-03/jul2020.pdf Accessed 2023 Jul 17
- 4Abdulbagi M, Wang L, Siddig O, Di B, Li B (2021) Damino acids and D-amino acid-containing peptides: potential disease biomarkers and therapeutic targets? Biomolecules. 11(11): 1716. doi.org/10.3390/biom11111716 Accessed 2023 Jul 18
- 5Truman R (2022) Racemization of amino acids under natural conditions: part 1—a challenge to abiogenesis. J Creation. 36(1): 114–121. https://dl0.creation.com/articles/p157/c15743/j36_1_114-121.pdf Accessed 2023 Jul 17
- 6Truman R (2022) Racemization of amino acids under natural conditions: part 2—kinetic and thermodynamic data. J Creation. 36(2): 72–80. https://www.researchgate.net/publication/369549082_Racemization_of_amino_acids_under_natural_conditions_part_2-kinetic_and_thermodynamic_data Accessed 2023 Jul 17
- 7Truman R (2022) Racemization of amino acids under natural conditions: part 4—racemization always exceeds the rate of peptide elongation in aqueous solution. J Creation. 36(3):74–81. https://www.researchgate.net/publication/365174403_Racemization_of_amino_acids_under_natural_conditions_part_4-racemization_always_exceeds_the_rate_of_peptide_elongation_in_aqueous_solution Accessed 2023 Jul 17
- 8Singh J, Whitaker D, Thoma B, Islam S, Foden CS, Aliev AE, Sheppard TD, Powner MW (2022) Prebiotic catalytic peptide ligation yields proteinogenic peptides by intramolecular amide catalyzed hydrolysis facilitating regioselective lysine ligation in neutral water. J Am Chem Soc. 144(23):10151−10155 https://doi.org/10.1021/jacs.2c03486 Accessed 2023 Jul 17
- 9Exceptions are known. For example, a protein that only contains lysine with only \(\epsilon\) linkages has been reported. See: Liu Y, Chen X, Pan L, Mao Z (2019) Differential protein expression of a streptomycin-resistant Streptomyces albulus mutant in high yield production of \(\epsilon\)-poly-L-lysine: a proteomics study. RSC Advances. 9:24092. https://pubs.rsc.org/en/content/articleanding/2019/RA/C9RA03156A Accessed 2023 Jul 17
- 10For example, see Figures S102 and S140 and the accompanying NMR results in the supplementary file for footnote 8, available at https://pubs.acs.org/doi/suppl/10.1021/jacs.2c03486/suppl_file/ja2c03486_si_001.pdf
- 11Canavelli P, Islam S, Powner MW (2019) Peptide ligation by chemoselective aminonitrile coupling in water. Nature. 571: 546−549. https://doi.org/10.1038/s41586-019-1371-4
- 12 a b Chandru K, Gilbert A, Butch C, Aono M, Cleaves HJ II. (2016) The abiotic chemistry of thiolated acetate derivatives and the origin of life. Sci Rep. 6: 29883. doi: 10.1038/srep29883. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4956751/pdf/srep29883.pdf.
- 13Thoma B, Powner MW (2023) Selective synthesis of lysine peptides and the prebiotically plausible synthesis of catalytically active diaminopropionic acid peptide nitriles in water. J Am Chem Soc. 145: 3121−3130.
- 14See Table 13 in the supplementary information file for reference 11.
- 15Bordier F, Stam M, Darii E, Tricot S, Fossey A, Rohault J, Debard A, Mariage A, et al. (2014) Large \(\alpha\)-aminonitrilase activity screening of nitrilase superfamily members: Access to conversion and enantiospecificity by LC–MS. J Mol Catal B: Enzym. 107:79–88. http://dx.doi.org/10.1016/j.molcatb.2014.05.019.
- 16For example, see Parker ET, Zhou M, Burton AS, Glavin DP, Dworkin JP, Krishnamurthy R, Fernández FM, Bada JL (2014) A plausible simultaneous synthesis of amino acids and simple peptides on the primordial Earth. Angew Chem Int Ed. 53(31): 8132–8136. https://doi.org/10.1002/anie.201403683.
- 17See the supplementary file for reference 11, S39-40.